Using Two Chemical Compounds as an Example Describe the Difference
A molecule is formed when two or more atoms of an element chemically join together. Use water as an example to contrast the properties of a compound with the elements from which it is composed.

Difference Between Empirical And Molecular Formula Infographic Chemistry Lessons Chemistry Study Guide Chemistry Education
An example of a covalently bonded compound that forms a.

. HCl CO2 H3NaCl NO2 P4 PCl5 C6H12O6 etc. For example we are taking NaCl. Covalent compounds metallic compounds and ionic compounds.
Linguistics A lexeme that consists of more than one stem. The compounds to be separated are treated. Hydrogen has a slightly positive charge and oxygen has a negative charge and therefore it forms a polar molecule.
The elements are connected with covalent or ionic bonds in the compounds. These elements are combined together in fixed proportions and structures to produce a single type of. Updated on December 10 2019.
A mixture is created when two or more substances are combined physically in indefinite proportions. Mixtures are substances that are formed by physically mixing two or more substances. An example of a chemical reaction is the burning of methane in the presence of molecular oxygen O 2 to form carbon dioxide CO 2 and water.
The key difference between compound and mixture is that the compound contains two or more components bound to each other by chemical means whereas the mixture contains two or more components bound to each other by physical means. Not all molecules are compounds because some molecules such as hydrogen gas or ozone consist only of one. Extraction literally taking out by force is a useful technique for separating compounds such as I 2 and KMnO 4 that have different polarities.
For example alkanes are composed of C-C single bonds. A compound is a substance formed when two or more elements chemically react with. An element is a simple substance that is made from one type of atom and cannot be broken down into simpler components by chemical or physical means.
For example laptop formed. As said earlier all compounds are molecules but not all molecules are compounds. By that bond they are combined and form sSodium cChloride NaCl.
This is to distinguish it from its aqueous solution. CH 4 2O 2 CO 2 2H 2 O In this reaction which is an example of a combustion reaction changes occur in the way that the carbon hydrogen and oxygen atoms are bound together in the compounds. The question is not surprising as the names substance and compound alone do not inherently convey the difference.
The Law of multiple proportions applies when two or more elementscompounds have multiple ways of combining into different compounds. The chemical formula of water molecule H 2 O. Chemistry dated A substance made from any combination elements.
Two distinct elements form a bond between their atoms and they are combined into a chemical compound. Nevertheless these two chemical species differ from. Start studying Describe the difference between elements compounds and mixtures and give three examples.
One of the most important and time-consuming activities in chemistry involves isolating separating and purifying chemical compounds. And a compound is a type of molecule in which the types of atoms forming the molecule are different from each other. A chemical reaction needs to be conducted to show the property.
Answer Law of Definite Proportions. A compound and a mixture both contain two or more components. A chemical compound is a chemical that is composed of at least two different types of elements.
Chemistry A substance formed by chemical union of two or more ingredients in definite proportions by weight. An example of an ionic compound that forms a crystal lattice is sodium chloride NaCl. A substance that can not be broken down into any other substances by an.
Cement is a mixture because it is made up of sand water and gravel. Anhydrous compounds are used for chemical processes where chemical reactions should be done in the absence of. A mixture is made from two or more substances that are chemically different and are not chemically joined.
Describe two ways in which atoms become stable by combining with each other. In other words a given compound will always contain the same elements in the same proportions by mass. Sometimes the term anhydrous is used to describe the gaseous phase of a compound.
An easy example would be salt which is a compound because it contains two elements sodium and chlorine. En noun Anything made by combining several things. H 2 O Hydrogen 2 Oxygen Two atoms of the element Hydrogen combine with one atom of Oxygen through a covalent bond to form water.
The chemical bond can be covalent or ionic or metallic. Using two chemical compounds as an example describe the difference between the lawof definite proportions and the law of. However the compound has no water molecules.
A saturated compound is only composed of carbon-carbon single bonds. In PubChem terminology a substance is a chemical sample description provided by a single source and a compound is a normalized chemical structure representation found in one or more contributed substances. Compounds can be of three types which are.
Organizing Ideas Using two chemical compounds as an example describe the difference between the law of definite proportions and the law of multiple proportions. A group of different atoms and different elements united together by the chemical bond is known as the compound. For example anhydrous ammonia is gaseous ammonia.
Two or more substances that are together in the same place but their atoms are not chemically combinedbonded-Examples. Sodium Na and chloride Cl come together and form an ionic bond. Compound are substances which can be formed by chemically combining two or more elements.
No chemical reaction is needed here. Electronegativity difference between the two elements involved in the bond. Physical properties are properties that can be observed without bringing a chemical change.
In organic chemistry a chemical compound can be either saturated or unsaturated based on the type of chemical bonding between carbon atoms. Compare and contrast the compounds that result from. Molar mass of water molecule 18 gmol.
For example either it is tap water or sea water a water molecule will always contain hydrogen and oxygen elements in the following proportions. Chemical properties are properties that can be observed or measured when a substance undergoes a chemical change. Sand guacamole and honey.
Water can be split back into hydrogen and oxygen through electrolysis. A chemical compound contains the same elements in exactly the same propotions by. These terms are to describe alkanes alkenes and alkynes.
The Law of Definite Proportions or Prousts Law states that in a single chemical compound such as H2O or CO the ratio of its component elements is a fixed whole number ratio. When a compound has chemical bonds that are mostly covalent in nature the structure that is formed is a molecule. Compounds can be classified as organic.
The chemical reactivity of compounds is LESS than that of the elements of which they are composed.
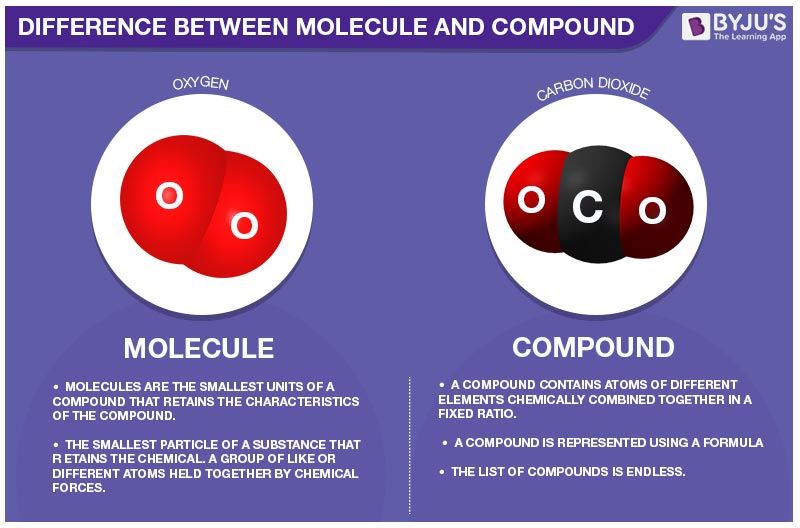
Difference Between Molecule And Compound In Tabular Form

Difference Between Compounds And Mixtures Structure Of Matter Cbse Grade 07 Chemistry Youtube

Difference Between Organic And Inorganic Compounds Definition Structure Properties Chemistry Lessons Chemistry Education Teaching Chemistry
Comments
Post a Comment